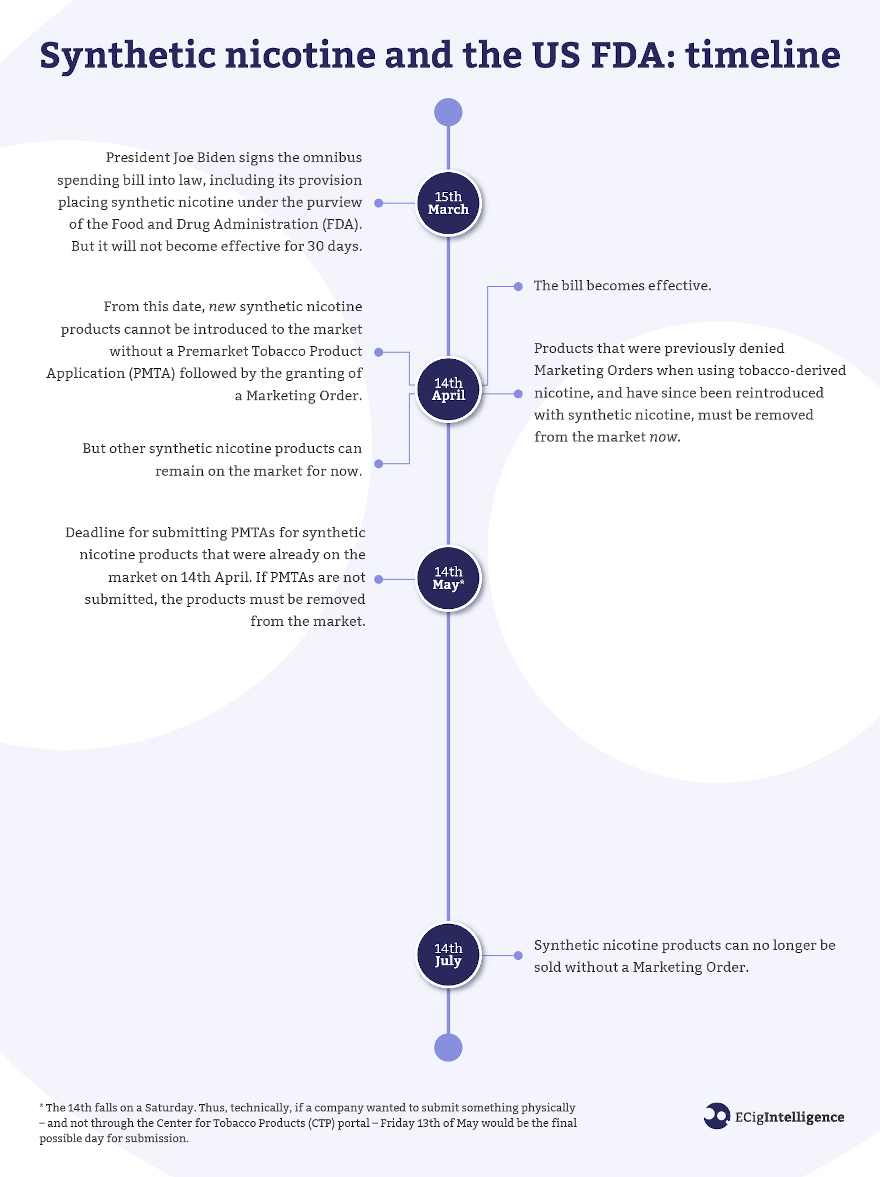
The passage of the $1.5tn omnibus spending bill and its rider giving the US Food and Drug Administration (FDA) authority to regulate synthetic nicotine products in the same way that tobacco products are regulated will irrevocably change the US vaping market.
Now that president Joe Biden has signed the bill into law, there are a number of major milestones to look out for over the coming months. ECigIntelligence has put together a timeline outlining the major points to look out for over the summer as the FDA brings synthetic nicotine into the PMTA process.
– ECigIntelligence staff






